- Purpose and Rationale
Many individual users contact the AAPM Brachytherapy Subcommittee (BTSC) and the IROC Houston QA Center asking which of the available photon-emitting brachytherapy source models conform to the AAPM’s guidelines. The AAPM guidelines are based on the following reports for low-energy (LE) and high-energy (HE) photon-emitting brachytherapy sources:
- Nath R, Anderson L L, Luxton G, Weaver K A, Williamson J F, and Meigooni A S. Dosimetry of interstitial brachytherapy sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No. 43, Med Phys. 1995; 22: 209–234.
- Nath R, Anderson L L, Meli J A, Olch A J, J. A. Stitt, and J. F. Williamson. Code of practice for brachytherapy physics: Report of the AAPM Radiation Therapy Committee Task Group No. 56. Med Phys.1997; 24: 1557–1598.
- Kubo H D, Glasgow G P, Pethel T D, Thomadsen B R, Williamson J F. High dose rate brachytherapy treatment delivery: Report of the AAPM Radiation Therapy Committee Task Group No. 59. Med Phys1998; 25: 375–403.
- Williamson J F, Coursey B M, DeWerd L A, Hanson W F, Nath R, Ibbott G. Guidance to users of Nycomed Amersham and North American Scientific, Inc. I-125 Interstitial Sources: Dosimetry and calibration changes: Recommendation of the American Association of Physicists in Medicine Radiation Therapy Committee Ad Hoc Subcommittee on Low-Energy Seed Dosimetry. Med Phys. 1999; 26:570–573.
- Williamson J F, B. M. Coursey, L. A. DeWerd, W. F. Hanson, R. Nath, M. J. Rivard, and G. Ibbott, ‘On the use of apparent activity (Aapp) for treatment planning of 125I and 103Pd interstitial brachytherapy sources: Recommendations of the American Association of Physicists in Medicine Radiation Therapy Committee Subcommittee on Low-Energy Brachytherapy Source Dosimetry. Med Phys. 1999; 26: 2529–2530.
- Rivard M J, Coursey B M, DeWerd L A, Hanson W F, Huq M S, Ibbott G S, Mitch M G, Nath R, Williamson J F. Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Med Phys. 2004; 31: 633–674.
- Rivard M J, Coursey B M, DeWerd L A, Hanson W F, Huq M S, Ibbott G S, Mitch M G, Nath R, Williamson J F. Erratum: Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations. Med Phys. 2004; 31 :3532-3533
- DeWerd L A, Huq M S, Das I J, Ibbott G S, Hanson W F, Slowey T W, Williamson J F, Coursey B M. Procedures for establishing and maintaining consistent air-kerma strength standards for low-energy, photon-emitting brachytherapy sources: Recommendations of the Calibration Laboratory Accreditation Subcommittee of the American Association of Physicists in Medicine. Med Phys. 2004;31: 675–681.
- Li Z, Das R K, DeWerd L A, Ibbott G S, Meigooni A S, Perez-Calatayud J, Rivard M J, Sloboda R S, Williamson J F. Dosimetric prerequisites for routine clinical use of photon emitting brachytherapy sources with average energy higher than 50 keV. Med Phys. 2007;34: 37–40.
- Rivard M J, Butler W M, DeWerd L A, Huq M S, Ibbott G S, Meigooni A S, Melhus C S, Mitch M G, Nath R, Williamson J F. Supplement to the 2004 update of the AAPM Task Group No. 43 Report. Med Phys. 2007; 34:2187–2205.
- Rivard M J, Butler W M, DeWerd L A, Huq M S, Ibbott G S, Meigooni A S, Melhus C S, Mitch M G, Nath R, Williamson J F. Erratum: Supplement to the 2004 update of the AAPM Task Group No. 43 Report [Med. Phys. 2007; 34,2187–2205. Med Phys. 2010; 37: 2396
- Butler W M, Bice Jr. B S, DeWerd L A, Hevezi J M, Huq M S, Ibbott G S, Palta J R, Rivard M J, Seuntjens J P, Thomadsen B R. Third-party brachytherapy source calibrations and physicist responsibilities: Report of the AAPM Low Energy Brachytherapy Source Calibration Working Group. Med .Phys. 2008; 35: 3860–3865.
- Pérez-Calatayud J, Ballester F, Das R K, DeWerd L A, Ibbott G S, Meigooni A S, Ouhib Z, Rivard M J, Sloboda R S, Williamson J F. Dose calculation for photon-emitting brachytherapy sources with average energy higher than 50 keV: Report of the AAPM and ESTRO. Med Phys. 2012;39:2904–2929.
- Rivard M J, Ballester F, Butler W M, DeWerd L A, Ibbott G S, Meigooni A S, Melhus C S, Mitch M G, Nath R, Papagiannis P. Supplement 2 for the 2004 update of the AAPM Task Group No. 43 Report: Joint recommendations by the AAPM and GEC-ESTRO. Med Phys. 2017; 44 e297–e338.
- Rivard M J, Ballester, Butler W M, DeWerd L A, Ibbott G S, Meigooni A S, Melhus C S, Mitch M G, Nath R, Papagiannis P. Erratum: Supplement 2 for the 2004 update of the AAPM Task Group No. 43 Report: Joint recommendations by the AAPM and GEC-ESTRO. Med Phys. 2018;45:971-974
The AAPM and IROC Houston work together to identify and register brachytherapy sources that meet the AAPM criteria for use at institutions participating in the NCI’s National Clinical Trial Network clinical trials. A webpage-based Registry is maintained by IROC Houston, listing sources that meet the AAPM criteria. IROC Houston relies on BTSC, through its Brachytherapy Source Registry Work Group (WGBSR), and the Calibration Laboratory Accreditation Subcommittee (CLA) to establish the criteria for including or excluding source models from this Registry, and to determine which source models meet the criteria.
As the recommendations are intended to apply internationally, some of the agencies, organizations and standard laboratories identified within the U.S. could be, at the discretion of the BTSC, interpreted in the context of the arrangements in other countries where applicable.
- Compliance, Registry, Appearance, and Contents
The Registry is in the form of a webpage reachable via hyperlinks from both the AAPM and IROC Houston websites, and is entitled “Joint AAPM/IROC Houston Registry of Brachytherapy Sources Meeting the AAPM Dosimetric Prerequisites.” The webpage (or links within this webpage) displays the following information for each source model listed on the Registry: The vendor (manufacturer if different), radionuclide, model number, trade name, date of addition to the Registry, and dosimetry publications taken as evidence of compliance.
Links to this Policy, the AAPM prerequisites publications, other publications subsequently determined to be relevant, as well as an application form to be used by source vendors are also kept accessible on the Registry webpage (or links within this webpage).
The text in Appendix 1 shall appear verbatim on the Registry webpage.
The “Application for Inclusion” and similarly “Application for Removal” in the Registry are available for download from the Registry webpage and also appears in Appendix 2 and Appendix 3, respectively.
- Procedure for Registry Inclusion of a Source
Figure 1 illustrates the flowchart of the procedure for Registry inclusion of a new source model.
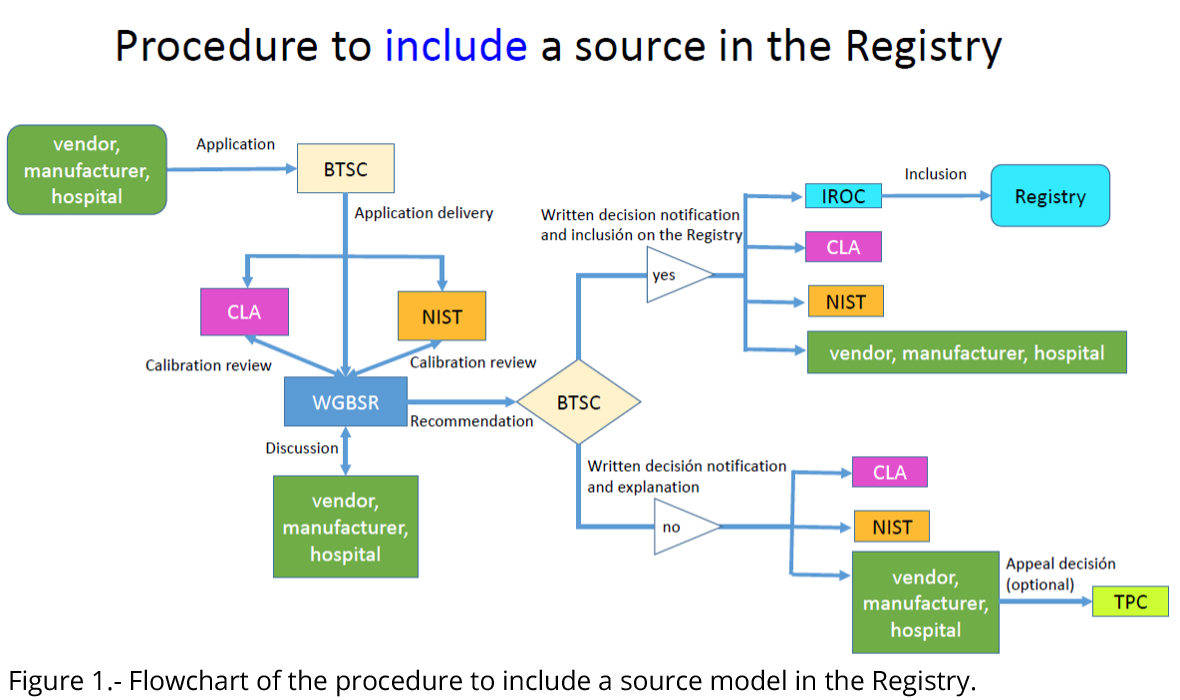
- Request for inclusion of a source model is initiated by the vendor/manufacturer/hospital (the applicant in the following) applying to the BTSC for this purpose.
- A special case exists for orphaned sources; those no longer commercially available, but still in regular use in hospitals. These must be sources with long half-life and suitable dose rates which consequently comprise only certain models of 137Cs and 60Co sources. In the case of these sources, there is no manufacturer/vendor available to submit the Registry application forms. For these orphaned sources, the AAPM and IROC Houston have agreed to an alternative procedure for Registry application: a hospital that wishes to participate in a clinical trial that involves brachytherapy sources not currently posted on the Registry may submit the application, listing the dosimetric studies available and the dosimetry parameters to be used for treatment planning. The hospital must also describe their method of source strength traceability for review by IROC Houston to assure the correct calibration of the sources. In the special case of source trains, in which individual sources cannot be removed for calibration with a well chamber, the hospital may describe a method of calibration at a distance in a phantom, in accordance with calibration procedures described in the peer-reviewed literature.
- Upon receipt, BTSC will deliver the application to WGBSR and the CLA Chair. WGBSR will review the published/accepted dosimetry papers and the applicant’s statements regarding calibration procedures with the NIST representative on the BTSC and the CLA chair.
- WGBSR may withhold entry into the Registry until any discrepancies between the applicant’s statements and calibration laboratory personnel observations are resolved satisfactorily. Following its review and a 2/3 majority vote, WGBSR will forward a recommendation for or against inclusion in the Registry to BTSC.
- BTSC has adopted the following “Independence Policy” (reproduced from “Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations.” Med. Phys. 31, 633-674 (2004):
The first meaning of “independent studies” is that they are performed, written, and published by investigators who are affiliated with institutions independent of the source vendor and who have no major conflicts-of interest with that vendor.
The second meaning of “independent studies” is that they are scientifically independent of one another, i.e., they represent independent and distinct estimations of the same quantities. In the case of two measurement-based studies, this will usually mean that two different investigators have used their own methodologies for measuring L and sampling the relative dose distribution, as TLD dosimetry is highly technique and investigator dependent. In the case of an empirical study and a Monte Carlo study, if properly executed, they will yield scientifically independent estimates of the TG-43 parameters. Thus, so long as the two studies are successfully scrutinized by the peer-review process and satisfy the AAPM scientific requirements, the empirical and Monte Carlo investigator author lists can overlap or even be identical. It is permissible to publish the Monte Carlo and measured estimates in the same paper so long as the two datasets are independently tabulated. In this context, “Not independent” means that the one study is used to modify the outcomes and methods of the other to improve agreement between the two datasets in a manner that is not scientifically justified.
- BTSC has adopted the following “Data Overlap Policy”:
We require that, for sources other than conventionally-encapsulated 137Cs, 192Ir, and 60Co sources similar in design to existing ones, two dosimetry studies each assess: (a) L; (b) gL(r) or gP(r); (c) F(r,q) and fan(r) with r and q sampling resolution at least as good as that recommended in the 2004 AAPM TG-43U1 (low-energy) and Li et al. 2007 (high-energy) reports so that overlap between the two studies may readily reveal significant discrepancies if present; and (d) L or Leff. As provided in reference (8) above, a single dosimetry study is acceptable for conventionally-encapsulated 137Cs 192Ir, and 60Co sources similar in design to existing ones.
- BTSC expects that manufacturers/vendors will comply with the AAPM prerequisite requiring periodic comparisons of their calibrations with those of an independent laboratory. Vendors can best do this by participating in comparisons with both NIST and the ADCLs offering calibration services for that source model. The AAPM has recommended a procedure and a frequency for such comparisons in the 2004 CLA Report. While the AAPM High Energy Brachytherapy Source Dosimetry working group (HEBD) has set a calibration comparison frequency of two years for 137Cs, 192Ir, and 60Co, this is not a requirement for manufacturers/vendors to comply with this AAPM registry due to the difficulties in circulating these sources to calibration laboratories, as well as the relatively insensitive nature of well chamber calibrations to small manufacturing changes.
- Inclusion into the Registry is by 2/3 majority vote of the BTSC.
- Upon completion of a positive vote for inclusion by the BTSC, applicant, NIST, CLA and IROC Houston will be informed of the decision and instructed to add the source model to the Registry.
- Written notification of any unfavorable decision along with an explanation will be provided to the applicant, NIST and CLA by the WGBSR chair.
- Upon receipt of an unfavorable decision, the applicant should provide a response within one month addressing all issues raised by the WGBSR, and the process starts again in step 2.
- Upon receipt of final notification of rejection of a source from the Registry, an applicant may appeal the decision by submitting a written request for review to the AAPM Therapy Physics Committee (TPC). The TPC will assign a minimum of three physicists to review the materials submitted to the BTSC and the decision made by BTSC. TPC may decide to endorse or reverse the decision of the BTSC.
- BTSC reserves the right to withhold entry into the Registry if it believes that the dosimetry articles contain technical errors or omissions; or rely on unproven or inappropriate computational or experimental dosimetry methodologies.
- This Policy may be revised as new source models or technologies emerge.
- Procedures for Registry Removal
Figure 2 illustrates the flowchart of the procedure for Registry removal of a new source model.
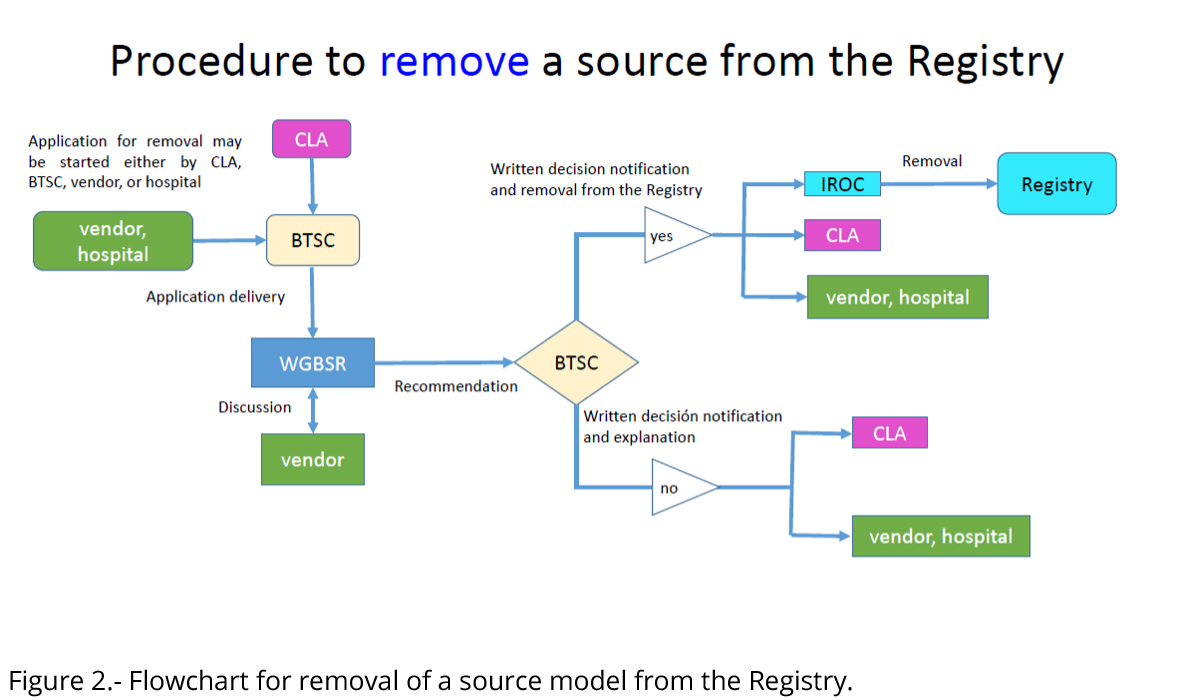
Manufacturer request to removal of a source
Manufacturer/vendors (or hospitals according to section C.1.a) may request that a source be removed from the Registry on the grounds that it will no longer be available to the medical community. Figure 2 displays the flowchart of the procedure to remove a source model from the Registry. Immediate steps will be taken to remove the source from the Registry:
- Upon receipt of a specific written request from the CLA, manufacturer, source distributor, or hospital, the BTSC will deliver it to the WGBSR.
- The WGBSR will review the request and contact the applicant when necessary.
- WGBSR will forward a recommendation for or against the removal of the source from the Registry to BTSC.
- Upon completion of a positive vote (2/3 majority) for removal by the BTSC, applicant, CLA and IROC Houston will be informed of the decision and instructed to remove the source model from the Registry.
- Written notification of any unfavorable decision along with an explanation will be provided to the applicant and CLA by the WGBSR chair.
Process to remove a source by failure of maintenance program of calibration comparison with NIST
Failure of a manufacturer/vendor to maintain a regular program of comparisons with NIST and an ADCL, in accordance with the recommendations of the AAPM Calibration Laboratory Accreditation (CLA) Subcommittee (Med. Phys. 2004;31:675-681 or Med. Phys. 2007; 34:37-40) may result in removal of the source from the Registry. NIST shall update the CLA chair at regular (at least quarterly) intervals as to the status of each seed vendors’ seeds’ calibration timelines. When the CLA chair determines that a vendor has exceeded the recommended interval, defined as one year since the last calibration report, the CLA chair will issue a warning via an email with an attached formal letter to the vendor. A template of this letter is included as an example in Appendix 4. The manufacturer/vendor is responsible for responding to this warning by scheduling a calibration at NIST within 60 days of receiving the warning letter. If this does not happen, then the CLA chair will notify the BTSC chair of the specific timeline and details of the situation.. The BTSC will review the situation and any decision to remove a source model from the Registry will be made by 2/3 majority vote of the BTSC in a closed executive session. If a 2/3 majority vote to remove a seed from the registry passes, the BTSC chair shall instruct IROC Houston to remove the source from the webpage Registry and shall notify the WGBSR chair of this action.
Process to remove a source due to changes in the source model
While manufacturers are encouraged to contact the BTSC should there be any changes in their source manufacturing processes, the BTSC retains the discretion to remove a product from the Joint AAPM/IROC Houston Registry should it have grounds to believe that a product, as currently manufactured, is no longer accurately characterized by the reference-quality dose-distribution data submitted in support of Registry posting. Reasonable grounds include but are not limited to: change of manufacturing venue or process, changes in source geometry and/or internal design, and discovery of deficiencies in the published dosimetry data.
Under these circumstances, the CLA chair will issue a warning via an email to the manufacturer with an attached formal letter to the vendor. A template of this letter is included as an example in Appendix 5. To retain Registry status, the manufacturer may submit for CLA review a set of revised dosimetry data that meet the standards specified by the AAPM dosimetric prerequisites. Alternatively, the vendor may provide evidence to CLA demonstrating that the revised product is dosimetrically equivalent to the original source from which the published and accepted dosimetry data were derived. Such information should include, as applicable,
- Assurances from the vendor that manufacturing processes are unchanged and the geometric and compositional structure of relevant source components are unchanged.
- Review of quality assurance data (e.g., source radiograph, optical micrographs, measurements, etc.) and vendor manufacturing processes with original dosimetry investigators. These investigators must make a recommendation regarding equivalence to BTSC and be willing to discuss findings with BTSC in closed session.
- Review of NIST anisotropy and spectroscopy measurements before and after the product change.
- A description of the vendor’s efforts and checks, along with their outcome, to assure the equivalence of their current product with that at the time of original dosimetric characterization.
The above information should be submitted to BTSC with sufficient lead-time that BTSC can make a recommendation prior to the vendor distributing the modified product commercially for routine clinical use.
Appendices
|






















