Abstract Submission
Annual Meeting Online Submission (AMOS) System Instructions
Welcome to the 2011 Joint AAPM/COMP Meeting Abstract Submission System
Abstract submission for the 2011 Joint AAPM/COMP Meeting is a 100% web based process. In order to complete a SUCCESSFUL SUBMISSION, understanding how the system works, what the requirements are, and what information is needed will streamline the process for you.
So prior to beginning the abstract submission process, please take a few minutes to review the information. With a general understanding, your submission should be successful.
Deadline Date: Monday, March 7 (5:00 PM EASTERN)
There will be NO EXTENSION OF THIS DEADLINE.
Authors must submit their abstracts by March 7 at 5:00 PM EASTERN
to be considered for review.
Items of Interest:
- NEW FOR 2011 SUBMISSIONS:
- ABSTRACT - Enter your abstract in the designated field. The abstract entry field is text only and does not support symbols or equations.
- There will be a tiered-down approach for selecting categories with major categories and tiered sub-categories.
- The Moderated Poster Category has been removed and will be replaced with a “Short Oral Presentation†featuring brief oral presentations (5 minutes + 1 minute Q/A) in a moderated session.
- The Scientific Program Committee STRONGLY SUGGESTS that the supporting documentation include a statement of Innovation/Impact to help identify the most exciting and interesting submissions.
- Innovations in Medical Physics Education Symposium
- Registration Fee Waivers for Presenting Authors from Developing Countries:
The AAPM will be awarding 10 registration fee waivers to PRESENTING AUTHORS who reside and work in developing countries recognized by the AAPM.
- Individuals who are interested in the potential registration fee waiver must complete the Registration Waiver Request Form and submit to HQ as instructed by MARCH 7.
- Preference is given to those abstracts which are accepted for Oral presentations.
- Only the PRESENTING AUTHORS (who reside and work in developing countries per the recognized AAPM list) of Oral presentations are eligible for the first round of the selection process for the registration fee waivers.
- If the number of eligible authors is more than 10, the AAPM International Affairs Committee (IAC) will select one from each region. The remaining awards will be selected by IAC members from the remaining eligible authors by ballot.
- Recipients of the registration fee waivers will be contacted on May 5, 2011.
Self Assessment Modules (SAMs): Completion of two Self Assessment Modules (SAMs) per year is a requirement for the Maintenance of Certification (MOC) process as defined by the American Board of Radiology (ABR). AAPM program organizers will identify sessions in both Diagnostic and Therapy Physics to be offered with audience response technology at the annual meeting in 2011. These sessions will allow those who require MOC to fulfill their SAMs requirements. Credits for these sessions are available only to attendees who pre-register for the modules. No SAMs registration will be offered on-site in Vancouver.
- Please note that the SAMs sessions will be open to everyone, but only those pre-registered for the audience response technology will be eligible to receive an interactive response unit. Questions will be asked during the SAMs sessions, and participants must answer electronically in order to receive certification. There is no pass/fail. The idea is to assess knowledge gained during the session.
- Meeting Registration will be available on-line: March 16, 2011.
General Rules and Guidelines
- Proffered Abstracts should be original work not previously presented or submitted to any other conference, UNLESS specific permission has been granted by the Scientific Program Directors
- Abstracts failing to meet the requirements detailed herein may be rejected. See detailed information below for additional instructions.
Submission Details and Authors
- Complete all information regarding the Abstract submission and contributing authors: Submission Type, Title, Subject Category, Requested Presentation Mode, Contributing Author Information, and Program Byline (Author Information, Institutions, Acknowledgements/Agreements, etc.).
- Funding sources, financial disclosures, and conflicts of interest should be listed in the designated field. The information provided should encompass all funding sources supporting the research and disclosures / conflicts of interest pertaining to any or all persons on the author list.
Abstract
- Enter your Abstract in the designated field. The Abstract is limited to 300 words and should be structured as Purpose, Methods, Results, and Conclusions.
- The abstract entry field is text only and does not support symbols or equations. It should not contain any graphs, figures, tables, images or other multimedia objects (these can be entered in supporting dcoment, see below).
- The Abstract should NOT include Title, author names, affiliations, or other identifying information
Support Document
- A Support Document is STRONGLY RECOMMENDED (but optional) for regular scientific abstracts and limited to 2 pages.
- A Support Document is REQUIRED for the following submission types:
- John R. Cameron – John R. Cunningham Young Investigator's Competition - a Supporting document submission is REQUIRED and limited to 4 pages.
- Jack Fowler Junior Investigator Competition - a Supporting document submission is REQUIRED and limited to 4 pages.
- John S. Laughlin Science Council Research Symposium - a Supporting document submission is REQUIRED and limited to 4 pages.
- Innovations in Medical Physics Education Symposium - a Supporting document submission is REQUIRED and limited to 2 pages.
- The Support Document should contain the title and supporting text as well as any equations, graphs, figures, and/or tables, but it should NOT contain author names, affiliations, or other identifying information. Preferred format is illustrated in the Sample Support Document.
Review the Sample Abstract and Sample Support Document prior to preparing your submissions.
Policy on Number of Submissions
- An individual can present up to TWO first-authored presentations at the meeting, although the individual's name may appear on more than two Abstracts.
- If a presenter has submitted several Abstracts for presentation as first author, the TWO highest-scoring Abstracts will be selected, and the other(s) will be rejected.
Before Submitting Your Abstract and Support Document
- Gather each Author's full name and email address.
- Gather each Author's institution name and full address.
- Determine your requested Presentation Mode (Scientific abstracts only).
- Determine the most relevant Subject Category. Definitions are available.
- The Abstract text is limited to 300 words.
- The Abstract text should contain text only and does not support symbols or equations.
- Support Documents:
- For regular abstracts: Strongly encouraged (but optional) and limited to 2 pages.
- For Young Investigator Symposium: Mandatory and limited to 4 pages.
- For Junior Investigator abstract: Mandatory and limited to 4 pages.
- For John S. Laughlin – Science Council Research Symposium: Mandatory and limited to 4 pages.
- For Innovations in Medical Physics Education Symposium: Mandatory and limited to 2 pages.
- Compose in Microsoft Word, Corel WordPerfect, ASCII Text or PDF files.
- Should contain Title but no author names or affiliations.
- Should include a statement of "Innovation/Impact" (see Sample Support Document).
- May contain graphs, figures, equations, tables and images. See Upload the Support Document.
- Preferred format is illustrated in the Sample Support Document.
- Identify sources of funding, financial disclosures, and conflicts of interest in the submission field entitled "Funding Support, Disclosures, and Conflict of Interest." This information is entered once for the entire abstract and should list funding sources pertinent to the research and encompass disclosures and conflicts of interest pertinent to all authors.
Presentation Modes Defined
Abstracts selected for presentation will be assigned as one of the following:
Oral Presentation
- Oral presentation (8 minutes + 2 minutes Q/A) during scientific sessions.
- Each scientific session room will be equipped with one digital projection system for single projection of Microsoft PowerPoint Presentations.
Short Oral Presentation
- Brief oral presentation (5 minutes + 1 minute Q/A) in a moderated session.
- Authors are to present a very concise description covering Introduction, Materials & Methods, Key Results, and Conclusion.
Poster Presentation
- Brief overview of poster given during designated standard poster session
- 4' x 4' hardcopy poster displayed in the Exhibit Hall.
Abstract Formatting Guidelines
Adhere to the following when preparing your Abstract. The abstract text MUST be structured as follows:
- Purpose:
- Methods:
- Results:
- Conclusion:
**Any abstract that does NOT conform with this structured format will be REJECTED.**
- Review the Sample Abstract and Support Document before preparing your submission.
- Abstracts must not exceed 300 words.
- Enter Abstract text directly in the designated field on the website. If you Copy/Paste from word processing software, be sure to proofread integrity of the text. Symbols and equations are not supported.
- DO NOT include Title, Author names/institutions, graphs, figures, tables, images or multimedia elements. Titles and Author information is entered elsewhere in the system and will be merged with the abstract file later in the process.
Abstract Review Criteria
The following criteria will be used in determining the abstract score. If a Support Document is included, it will be used as additional information in determining the score.
- Clarity
- Quality and rigor of supporting data
- Significance
- Innovation and/or scientific impact
- Timeliness
- Interest to the medical physics community
Accessing the Submission System
Once you have accessed the System Site (AMOS), in order to access the submission system, you'll be prompted to login using an Abstract Submission Username and Password.
- If you have not submitted an abstract for this meeting, enter your email address and press ”submit”. Enter your First and Last Name and press “send it!” to have the system email you a temporary Username and Password.
- If you have forgotten your assigned Abstract Submission System Username or Password, enter your email address and press “submit” to have the system email the information.
- Once you obtain a username and password, you can access the submission system and will be taken to your personal Homepage for the meeting.
Personal Homepage
What information is available to me on the homepage?
- This page will have your address information, and information on any submissions that you create or that have been submitted on your behalf.
- The type of submission is shown next to the Title.
- The status of each submission is shown (Incomplete or Complete).
What can I do from my homepage?
- You may edit your personal information at any time including your username and password.
- If submission is open, you may create a new submission.
- You may edit any submissions where you are listed as the Corresponding Author.
- For submissions where you are listed as a Presenting or Contributing Author only, you will be able to view, but not edit the submission.
How do I create a new submission?
- Next to the meeting name, there is a link to "create new submission", click this link to begin the submission process.
- The steps for submitting an abstract are outlined in the next tab.
Instructions to Authors
To Create a New Submission
The following step by step instructions should be followed once you have accessed the abstract system to begin the submission process.
Review these instructions before you begin. You will need to follow these instructions precisely to successfully create and submit your abstract.
Expand All Steps | Collapse All Steps
Identify the submissions as one of the following:
- Proffered submission
- Young Investigator Symposium
- Junior Investigator submission
- John S. Laughlin – Science Council Research Symposium
- Innovations in Medical Physics Education Symposium
J.R. Cameron - J.R. Cunningham Young Investigator Symposium
Each year the AAPM conducts a Young Investigators' Symposium (YIS) competition at the Annual Meeting. This year the Young Investigators Symposium will be held jointly between the AAPM and the COMP. Young Investigators, as defined below, are encouraged to submit abstracts for the competition. The 10 highest scoring Young Investigator submissions as determined by the abstract reviewers will be selected for presentation in a special symposium in honor of University of Wisconsin Professor Emeritus John R. Cameron, Ph.D., and University of Toronto Professor Emeritus John R. Cunningham, Ph.D.
A panel of judges will score the oral presentations according to criteria that include scientific merit, originality, and organization/presentation of the material. Awardees will be announced at the Awards Ceremony on Monday night during the Annual Meeting.
If you wish to submit an abstract for the Young Investigator Symposium competition, you must identify the abstract as such. All instructions above apply to Young Investigator (YI) submissions with the exception of the Support Document, which is REQUIRED and must not exceed 4 pages (or 1 MB) in length.
Young Investigators are not eligible for the John S. Laughlin Science Council Research Symposium.
All abstracts submitted to the YIS Competition that are NOT selected for the competition, will be considered for oral, short oral, or poster presentation.
A Young Investigator is defined as a current graduate student at the time of abstract submission.
FINALISTS will be required to submit a letter of eligibility from the presenting author's thesis advisor identifying the institution. The letters are not to be submitted until the FINALISTS are identified in April, at which time the Finalists will be contacted with further instructions. Contact Laurie Hayden at AAPM HQ with any questions regarding the Young Investigator Competition.
Only one submission from each Young Investigator can be submitted for consideration for the Young Investigator Award competition. (Note, however, the Oral Presentation Policy above).
NOTE: The winner of the Young Investigator Award Competition is not allowed to participate in the Young Investigator Award Competitions of future AAPM Annual Meetings.
Jack Fowler Junior Investigator Submissions
An award for junior investigators has been established in honor of Dr. Jack Fowler, Emeritus Professor of Human Oncology and Medical Physics, University of Wisconsin.
Junior Investigators, as defined below, are encouraged to submit abstracts for the competition. The top scoring Junior Investigator submission determined by abstract reviewers will be selected and announced at the Awards Ceremony during the Annual Meeting.
If you wish to submit an abstract for the Junior Investigator competition, you must identify the abstract as such.
All instructions given above apply to Junior Investigator (JI) submissions with the exception of the Support Document, which is REQUIRED for JI submissions and must not exceed 4 pages (or 1 MB) in length.
Junior Investigators are eligible for the John S. Laughlin Science Council Research Symposium.
All abstracts submitted to the JI Competition that are NOT selected as the winner, will be considered for oral or standard poster presentation.
A Junior Investigator is defined as one of the following. (The junior investigator must be an APPROVED member of the AAPM at the time of abstract submission):
- Current medical physics resident, OR
- Current postdoctoral fellow, OR
- Staff/faculty member who is within 4 years of having obtained a graduate degree (at the time of abstract submission).
The AWARDEE will be required to submit a letter of eligibility from a Full Member of the AAPM at the junior investigator's sponsoring institution. The letter is not to be submitted until the Awardee is identified in April. Contact Laurie Hayden at AAPM HQ with any questions regarding the Junior Investigator Competition.
Only one submission from each Junior Investigator can be submitted for consideration for the Junior Investigator competition. (Note, however, the Oral Presentation Policy above).
John S. Laughlin - Science Council Research Symposium
A topic of particular relevance in medical physics research will be identified each year, with proffered submissions on that topic considered for inclusion in a scientific symposium entitled the John S. Laughlin Science Council Research Symposium. Abstracts selected for the Symposium will be highlighted in the scientific program and given an extended presentation length.
The topic selected for the 2011 Symposium is: Science and Engineering for Patient Safety in Therapy and Diagnostic Medical Physics
The 2011 Laughlin Symposium will focus on cutting-edge methods or devices designed to enhance, quantify, and/or monitor the safe use of radiation in diagnostic and therapeutic medicine, with emphasis on innovative science and engineering for patient safety. The timeliness of such work is evident in sensational coverage in the lay press reporting of accidents involving radiation in both the diagnostic and radiation therapy sectors. As a forum for high-quality, innovative scientific research, the symposium seeks abstract submissions on topics that go beyond basic quality control standards in radiation safety and identify new avenues for assuring patient safety.
Proffered submission: Authors interested in being considered for the Symposium MUST:
- Select the submission type: Science Council Research Symposium
- Select the subject category: Joint: Science and Engineering for Patient Safety in Therapy and Diagnostic Medical Physics
- Select Oral presentation mode
- Submit a Support Document not to exceed 4 pages in length.
Innovations in Medical Physics Education Symposium
The Education Council of the AAPM is sponsoring a symposium to honor and publicize innovations in Medical Physics Education. AAPM members are invited to submit a one page description of innovative medical physics educational activities for radiology residents, radiation oncology residents, medical physicists, technologists or others. The abstract can be scientific research, novel teaching strategies – team teaching or adult learning efforts, novel educational materials – lectures, websites, or other innovations.
The top six submissions will be invited to present their abstracts at the symposium during the annual meeting in Vancouver, BC. Each speaker will be allocated 15 minutes. The top abstract will be presented a plaque.
Proffered submission: Authors interested in being considered for the Symposium MUST:
- Select the submission type: Innovations in Medical Physics Education Symposium
- Select the subject category: Innovations in Medical Physics Education
- Select Oral presentation mode
- Submit support documentation not to exceed 2 pages in length.
All abstracts submitted to the Innovations in Medical Physics Education Symposium that are NOT selected for the symposium, will be considered for standard poster presentation.
Enter the title of the abstract to be submitted.
- Use sentence case when entering title.
- To enter superscript text put the following tags around the text to be superscripted: <sup>text</sup>
- To enter subscript text put the following tags around the text to be subscripted: <sub>text</sub>
Select the Subject Category which best pertains to your Abstract. Category Definitions are available here.
Rules regarding Requested Presentation Mode for Abstracts:
- Authors should request either Oral or Poster presentation mode.
- Final presentation mode assignments will be made by the Program Committee.
- Submissions requesting Oral Presentation may be assigned as ANY of the following:
- Oral presentation
- Short Oral presentation
- Poster presentation
- Any submission may be rejected based on the reviews by at least 3 referees.
Presentation Modes Defined:
Oral Presentation:
- This category entails a short presentation followed by a question/discussion period. NOTE: Single LCD projection will be the ONLY presentation method available.
- Details will be provided in abstract acceptance notification.
- Abstracts will be published in Medical Physics
Short Oral Presentation:
- Short oral sessions will consist of short presentations of approximately 5 minutes in length.
- Presentations will consist of a concise introduction, materials & methods, results, and conclusion.
- Sessions with moderators in a manner similar to standard oral sessions.
- Abstracts will be published in Medical Physics
Poster Presentation:
- Posters must be displayed for the entire meeting.
- The poster display will consist of a (4' x 4') poster. The Poster display may be a presentation of twelve 8.5 x 11" pages posted in the space provided.
- Pushpins will be provided, but authors may wish to use Velcro strips for mounting poster elements. (Use the hooked side of the Velcro strips on the back of your poster).
- Abstracts will be published in Medical Physics.
NOTE: The individual names of contributing authors MUST appear on all posted presentations.
On the Abstract Submission Status screen, you will be prompted to add any contributing author(s) to your submission.
To Add Author(s):
- Select the link to 'add an Author' and search for an individual's last name.
- A list of potential authors will be generated if the 'last name' is in the system.
- Select the correct contributing author for the submission and indicate if 'presenting' or 'author'.
- Continue process until all contributing authors have been added.
- Each submission is allowed only ONE presenting author.
- Corresponding authors are NOT automatically added to the submission as a contributing author. If the corresponding author is a contributing author, you MUST add the individual at this point.
If a Contributing Author is NOT currently in the System:
- Once you have searched for the last name and it does not appear on the generated author list, select 'enter a new author' and proceed through the process of creating a new record for the individual.
- By entering a unique email, each author will have access to the abstract submission content, but ONLY the corresponding author may edit the content.
- Use proper capitalization when entering a new author.
- Check your entry as you go as the system does not correct spelling or grammar.
Helpful Hints:
- Add your 'presenting author' first. You can later use the arrow buttons at left of names to change order, once all authors have been added.
- Finally, arrange the author names in the order they are to appear when published in the "Program By-Line" and "Calendar of Events By-Line." ANY AND ALL CONTRIBUTING AUTHORS MUST BE ADDED IN THIS SECTION OF THE PROCESS IN ORDER TO GENERATE THE CORRECT 'BY-LINES'. (see Steps 7 and 8).
Back on the Submission Status Page:
- The system will have generated a list of contributing authors added to your submission.
- If any contributing author's names are missing, go back and add the individuals.
- If the order of names is NOT correct, go back and use the arrow buttons to rearrange.
- The presenting author's name will be denoted with an asterisk.
- Any time you add/remove/reorder author names or reassign the presenting author, the system will change the content listed in Steps 6, 7, and 8 so it is IMPORTANT you review the content with each change made.
On the Abstract Submission Status screen, you will be prompted to view the 'Program By-Line' (Authors/Institutions) generated by the system. It is IMPERATIVE that the Program By-Line be VIEWED and EDITED as instructed.
What the System Generates:
- For a single author, the system will list author's name (first initial, last name), institution, city, and state.
- If multiple authors have been entered, the system will list ALL author names (first initials, last names) in the order of entry, followed by the first author's institution, city, and state, then the second author's institution, city, and state, etc.
To Edit the By-Line:
If all authors are from the SAME INSTITUTION:
- The final/correct By-line should list ALL AUTHOR names (first initials, last names) followed by ONE listing of the institution, city, and state.
- YOU MUST REMOVE all additional listings of institution, city, and state in order for the byline to be correct.
- YOU MUST SELECT the check box 'All authors are from the same institution' in order to proceed back to your status page or you will be forced to add super/sub-script coding, that is not necessary.
- Do not include "USA" or zip codes. Do not include departmental information.
- Do not abbreviate institution names.
--Use the following format when all authors are from the same institution:
T Webster*, M Warden, L Salliman, A Geyser, Memorial Sloan Kettering, New York, NY
(NOTE: The Presenting Author's name will be denoted with an asterisk.)
If authors are from MULTIPLE INSTITUTIONS:
- The final/correct By-line should list ALL AUTHOR names (first initials, last names) followed by EACH AUTHOR'S institution, city, and state.
- YOU MUST ADD 'super/sub-script coding' (<sup>1</sup>; <sup>2</sup>, etc) in order to correlate the correct Author with the correct institution, city, state.
- Do not include "USA" or zip codes. Do not include departmental information.
- Do not abbreviate institution names.
--Use the following format to add the required 'super/sub-script coding' when Authors are from multiple institutions:
Note that this code:
T Webster*<sup>1</sup>, M Warden<sup>1</sup>, L Salliman<sup>2</sup>, A Sullivan<sup>3</sup>, (1) Memorial Sloan Kettering, New York, NY, (2) University of Maryland, College Park, MD (3) UT M.D. Anderson Cancer Center, Houston, TX
--Produces this output:
T Webster*1 , M Warden1 , L Salliman2 , A Sullivan3 , (1) Memorial Sloan Kettering, New York, NY, (2) University of Maryland, College Park, MD, (3) UT M.D. Anderson Cancer Center, Houston, TX
Use of Reset Button:
- Anytime you make changes to the Author List, the system will require that the By-line be reviewed again. The system will display the former By-line, before changes were made.
- If you want to system to regenerate the By-line with the new authors/changes, hit the RESET BUTTON and make appropriate edits as listed above.
- The RESET BUTTON will always clear and reset to the most current author information.
- The 'Program By-Line' is EXACTLY what will be published in Medical Physics with the Abstract:

The corresponding author must indicate if the submission is to be considered for the John S. Laughlin Science Council Research Symposium.
- If YES, the submission subject category MUST be: Joint: Science and Engineering for Patient Safety in Therapy and Diagnostic Medical Physics
The corresponding author must agree to and select the following "Verification of Contributing Authors" statement button in the submission process:
"By checking this box, I verify that each author to be listed on the submitted abstract has contributed to the content of the abstract and consented to the submission of the said abstract."
To avoid the appearance of any conflict of interest, Authors of scientific presentations must disclose the names of any companies or organizations who have provided the Author with any financial support for the research being presented and whose products and/or services are the subject matter of the presentation. Such disclosure is to be made via the field entitled "Funding Sources, Disclosures, and Conflicts of Interest." Information is to be entered once per abstract and should cover all sources of funding pertaining to the research and financial disclosures and conflicts of interest pertaining to any author listed on the abstract.
This policy is not intended to discourage such support or restrict the dissemination of the research as it is recognized that much scientific research is supported by organizations that have a commercial interest in the results of the research. The intent of this policy is to permit the members of the audience to form their own judgments about the research with the full disclosure of the facts.
You will be prompted to acknowledge if any company or organization whose products/services are the subject matter of your abstract has provided you with any financial support for your research.
If your abstract submission is accepted for Oral, Short Oral, or Poster presentation, the authors must include an acknowledgment of funding sources, financial disclosures, and conflicts of interest within their presentations slides and/or poster.
The corresponding author must agree to and select the following "AAPM Participation Agreement" statement button in the submission process:
"By checking this box I verify that each author listed on the abstract has agreed to participate in this educational activity. It is understood that each presentation will be constructively evaluated in areas of presentation quality, achievement of educational objectives, and utility/usefulness of content. The evaluation will be confidential and communicated only to the contributing authors upon request.
I further verify that any potential conflict of interest(s), as set forth in the preceding statement, has been listed in the abstract and will be disclosed during the presentation."
The corresponding author must agree to and select the following "Distribution of Abstract and Presented Materials Agreement" statement button in the submission process:
"By checking this box, I verify that I have obtained permission from each author listed on my abstract allowing me to distribute the abstract at the AAPM meeting, via the AAPM website, or via any other publication that may result from this meeting.
Under this constraint, I verify that all contributing authors will obtain or have obtained permission for the use of any copyrighted materials that may be presented at the AAPM Annual Meeting for educational purposes from the appropriate copyright owner(s) and publisher(s).
Non-copyrighted materials obtained from sources such as colleagues or the web will be attributed accurately. Where possible, prior permission for its use will be obtained."
Corresponding Author Contact Information is entered on this screen. If you are an AAPM member, first log into the AAPM website. The following required fields will automatically be completed for you. The following fields are required:
- First Name
- Last Name
- Phone
- Street Address
- City/State/Zip
- Country
All Contributing Authors may access an Abstract by using their personal Username and Password. BUT only the Corresponding Author has the ability to modify any of the Abstract information, including withdrawing the abstract or re-submitting a revised Support Document.
You will be given the opportunity to withdraw the submission at this point.
If you choose to do so, this record will be deleted from the system. You would need to return to your private Abstract Homepage in order to 'create a new submission.'
- Review the Sample Abstract before preparing your submission.
- The Abstract text MUST be structured as follows:
- Purpose:
- Methods:
- Results:
- Conclusion:
**Any abstract that does NOT conform with this structured format will be REJECTED.**
- Abstracts must not exceed 300 words.
- Enter Abstract text directly in the designated field on the website. If you Copy/Paste from word processing software, be sure to proofread integrity of the text. Symbols and equations are not supported.
- DO NOT include Title, Author names/institutions, graphs, figures, tables, images or multimedia elements. Titles and Author information is entered elsewhere in the system and will be merged with the abstract file later in the process.
Once Steps 1 - 14 have been completed, the system will allow you to advance to the next screen to upload your Support Document.
Note: The Support Document should include a statement of Innovation/Impact to help identify the most exciting and interesting submissions.
- A Support Document is STRONGLY ENCOURAGED, but optional, for regular submission and is limited to 2 pages.
- A Support Document is REQUIRED for Young Investigator Competition submissions and limited to 4 pages.
- A Support Document is REQUIRED for Junior Investigator Competition submissions and limited to 4 pages.
- A Support Document is REQUIRED for John S. Laughlin Science Council Research Symposium submissions and limited to 4 pages.
- Preferred format is illustrated in the Sample Support Document.
- The Support Document may be submitted as Microsoft Word, Corel WordPerfect, ASCII Text format, or a PDF file and may contain graphs, figures, tables and images.
- The Support Document should include the title and may include text, symbols, equations, figures, images, tables, and references.
- In support of our blind review policy, there is to be no author information in the Support Document nor any information identifying the authors or affiliation.
- The Support Document must be uploaded to our server by clicking the "Upload Support Document" icon that will appear on your status page once you have provided all the required information.
- If submitting a PDF file, you must check the "pdf" box on the upload page.
- If not submitting a PDF file, the documents must be in Microsoft Word, Corel WordPerfect, or ASCII Text format. The recommended format, to ensure the highest level of compatibility, is Microsoft Word (97 or 2000).
- For best results, Mac WordPerfect users should save documents as MS Word 4, 5, or 6 prior to submission.
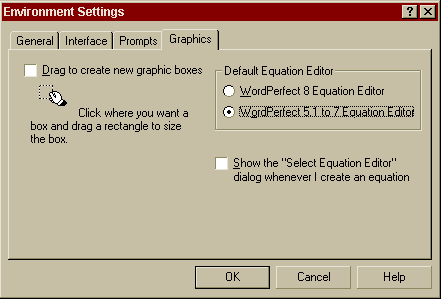
Special Note to WordPerfect 8 and higher users! Corel WordPerfect 8 and higher includes two equation editors. The WordPerfect 5.1-7 Equation Editor must be used when creating equations. To use the WordPerfect 5.1-7 Equation Editor, click Tools / Settings / Environment / Graphics / WordPerfect 5.1-7 Equation Editor.
POWERPOINT PRESENTATIONS:
For submission types where PowerPoint is accepted, ensure that the file does not exceed 2 pages (4 pages for Young and Junior Investigators) or it will automatically be rejected. Alternatively, create a PDF from your Support Document and submit the PDF.
This shows the status of the documents.
- By looking at this, the author should be able to see if the system has received the Support Document.
- If the information was received without any problems, there will be a link to the Adobe Acrobat PDF file that was created.
- Click on the "View Support Doc" buttons to view the document and ensure there were no errors introduced in conversion.
NOTE: If you previously submitted a document and would like to resubmit it for any reason, you may press the "Delete Support Doc" button. This will 1) notify the system that the document will be resubmitted and 2) all references to previously submitted documents will be removed. After resetting a document, resubmit the new document through the same web-based upload process.
You will only have the option to reset a submitted document if it has been received as noted above.
Please be completely sure that you wish to resubmit a document before following this procedure.
Submission Categories
Expand All Categories | Collapse All Categories
| Category | Description | |
|---|---|---|
| Innovations in Medical Physics | ||
| Submit a description of innovative medical physics educational activities for radiology residents, radiation oncology residents, medical physicists, technologists or others. The projects can be scientific research, novel teaching strategies – team teaching or adult learning efforts, novel educational materials – lectures, websites, or other innovations. | ||
| Category | Description | |
|---|---|---|
| CAD | ||
| New Technology and Techniques | Topics in computer-aided diagnosis, including new methodologies as applied to mammography, chest, CT and other modalities. | |
| Clinical Applications | Clinical application and/or evaluation of novel CAD technology and techniques. | |
| Computed Tomography | ||
| Reconstruction Techniques | Topics in all aspects of CT reconstruction, including cone-beam CT reconstruction, new reconstruction techniques, methods for fast reconstruction, correction of image artifacts, etc. | |
| Multi-Detector CT | Topics related to the design and performance of multi-detector CT imaging technology (e.g., 64-slice CT scanners), including detector design, system evaluation, imaging performance, and dosimetry. | |
| Cone-Beam CT (IMAGING) | For abstracts with primary focus in diagnostic imaging applications. Topics related to cone-beam CT, including the use of flat-panel detectors for cone-beam CT. Research related specifically to cone-beam CT reconstruction may be submitted in this subcategory or in "Reconstruction Techniques," above. | |
| Cone-Beam CT (JOINT) | For abstracts with primary focus in guidance of treatment delivery (e.g., localization in IGRT) or treatment planning. Topics related to cone-beam CT, including the use of flat-panel detectors for cone-beam CT. Research related specifically to cone-beam CT reconstruction may be submitted in this subcategory or in "Reconstruction Techniques," above. | |
| Dual-Energy and Spectral CT | Topics related to dual energy CT and spectral CT technology and methods. | |
| Clinical Applications | Clinical application of novel computed tomography technology and techniques. | |
| Display and pacs | ||
| Display Technology and Evaluation | Techniques for display of image data, including display performance evaluation, new display technologies, 2D and 3D displays, and related topics. | |
| Dosimetry, Radiation Protection, and Quality Control (Imaging) | ||
| Quality Control | Technology and techniques for imaging quality assurance, including topics related to the practice of diagnostic medical physics and radiology quality assurance (TQM, etc.). Included in this subcategory are topics of accreditation and regulatory interest, such as the ACR, FDA, etc. | |
| Imaging Dosimetry and Radiation Protection | All topics in the area of radiation protection, including shielding, safety, and dosimetry as related to medical imaging, including internal and external radiation sources. Special attention will be paid to submissions on new protection or dosimetry approaches. Abstracts concerning a given modality but focusing on dosimetric aspects (e.g., imaging dose in CT or PET) should be submitted under this category. | |
| Fluoroscopy | ||
| Fluoroscopy (IMAGING) | For abstracts with primary focus in diagnostic or interventional radiology applications. Topics related to real-time fluoroscopy, including digital techniques, imaging system design and characterization, new methods of performance characterization, and clinical studies. | |
| Fluoroscopy (JOINT) | For abstracts with primary focus in application to radiation treatment delivery (e.g., IGRT) or motion management applications (e.g., therapy planning). | |
| Image Registration, Fusion, Segmentation, and Visualization | ||
| Image Registration and Fusion (IMAGING) | For abstracts with primary focus in diagnostic imaging applications. Techniques for registration and/or fusion of image data, including any combination of (2D, 3D, and/or 4D) imaging modalities, deformable modeling, and related topics. | |
| Image Registration and Fusion (JOINT) | For abstracts with primary focus in treatment planning and guidance (e.g., IGRT). Techniques for registration and/or fusion of image data, including any combination of (2D, 3D, and/or 4D) imaging modalities, deformable modeling, and related topics. | |
| Image Segmentation (IMAGING) | For abstracts with primary focus in diagnostic imaging applications. Topics in all areas of medical image processing including new methodologies, image or feature enhancement, tissue segmentation, and clinical evaluation, as applied to any medical imaging modality. | |
| Image Segmentation (JOINT) |
For abstracts with primary focus in treatment planning and guidance (e.g., IGRT). Topics in all areas of medical image processing including new methodologies, image or feature enhancement, tissue segmentation, and clinical evaluation, as applied to any medical imaging modality. | |
| Image Visualization (IMAGING) |
For abstracts with primary focus in diagnostic imaging applications. Techniques for rendering and display of medical image data. | |
| Image Visualization (JOINT) |
For abstracts with primary focus in treatment planning and guidance (e.g., IGRT). Techniques for rendering and display of medical image data. | |
| image science | ||
| Imaging Performance: Measurement and Modeling | Topics of fundamental image science and imaging physics, including measurement and mathematical modeling of detector performance, imaging system evaluation, linear systems analysis, new evaluation techniques, performance metrology, etc. Topics specific to a given modality may be submitted in this category or that of the modality, at the authors' discretion. | |
| Observer Performance: Measurement and Modeling | Topics related to model and human observer performance, including experimental and theoretical analysis of observer performance (ROC, LROC, FROC, 2AFC, MAFC, etc.) and analytical models of human or machine visual systems. | |
| Magnetic Resonance Imaging | ||
| New Technology and Techniques (IMAGING) | All aspects of diagnostic MRI, MRS and MRA, including image acquisition, reconstruction, new technologies, and clinical studies. Unless specifically related to MR, abstracts concerning image processing, display, CAD, etc. should be submitted under respective categories, below. | |
| New Technology and Techniques (JOINT) | Abstracts related to the development or application of MRI, MRS, and/or MRA in radiation therapy planning and/or delivery. | |
| mammography | ||
| New Technology and Techniques | All aspects of breast imaging including digital x-ray mammography, tomosynthesis, CT, MRI, ultrasound, radioisotope imaging, optical and multi-Modality. Topics include novel detectors and their evaluation, instrumentation, system evaluation, and new techniques in mammography. Abstracts concerning image processing, display, CAD, etc. should be submitted under respective categories, below. | |
| Clinical Applications | Clinical application and/or evaluation of novel breast imaging technologies and techniques. | |
| Multi-Modality Imaging | ||
| CT + Nuclear Medicine | Imaging techniques combining CT and nuclear medicine (e.g., PET) techniques. Abstracts concerning image fusion may be submitted in this categories related to image registration / fusion. Abstracts concerning display, CAD, etc. should be submitted under respective categories, below. | |
| MR + Nuclear Medicine | Imaging techniques combining MR and nuclear medicine (e.g., PET) techniques. Abstracts concerning image fusion may be submitted in this categories related to image registration / fusion. Abstracts concerning display, CAD, etc. should be submitted under respective categories, below. | |
| X-Ray + Ultrasound | Imaging techniques combining x-ray and ultrasound techniques. Abstracts concerning image fusion may be submitted in this categories related to image registration / fusion. Abstracts concerning display, CAD, etc. should be submitted under respective categories, below. | |
| Other | Imaging techniques combining two or more modalities other than CT + Nuclear Medicine or X-ray + Ultrasound. | |
| non-conventional Imaging | ||
| All modalities with application in medical imaging not covered in other categories, including infrared imaging, impedance imaging, thermoacoustic imaging, MEG, and other topics. | ||
| nuclear medicine | ||
| New Technology and Techniques | All aspects of Nuclear Medicine, including SPECT and PET, imaging system design, image acquisition, reconstruction, tracers, and clinical studies. Abstracts concerning image fusion of nuclear medicine images should be submitted in the Visualization and Image Fusion category. Those concerning image processing, display, CAD, etc. should be submitted under their respective categories. | |
| Optical and other Novel imaging | ||
| All aspects of optical imaging, including optical coherence tomography, microscopy, detectors, lasers, image acquisition, reconstruction, and clinical studies. Abstracts specific to small animal imaging by optical methods may be submitted in this category or the Small Animal Imaging category. Other novel imaging techniques include electrical impedance imaging, microwave imaging, biomagnetic imaging, and other "cutting edge" imaging technologies. | ||
| radiography | ||
| New Technology and Clinical Applications | Topics related to x-ray radiography, including DR and CR, detector design and characterization, image acquisition techniques, tomosynthesis, and system design. They also include issues related to image acquisition and formation, e.g. scattered radiation, glare, etc. Abstracts concerning image processing, display, CAD, etc. should be submitted under respective categories, below. | |
| Small Animal Imaging | ||
| Abstracts related to preclinical imaging of small animals in biomedical research, including any imaging modality (e.g., CT, MR, PET, etc.). | ||
| Ultrasound | ||
| New Technology and Clinical Applications | Authors are directed to submit abstracts related to Ultrasound Imaging under the ULTRASOUND (SPECIAL CONFERENCE) track detailed above. All aspects of ultrasound imaging system design, image acquisition, reconstruction, and clinical applications. | |
| Vascular, Endovascular, and Cardiac Imaging | ||
| New Technology and Clinical Applications | Topics related to vascular, endovascular, and cardiac x-ray imaging. Abstracts on other modalities, including ultrasound, MRI, PET, etc., should be submitted in their respective categories. | |
| Category | Description | |
|---|---|---|
| John S. Laughlin / Science Council Research Symposium: Science and Engineering for Patient Safety in Therapy and Diagnostic Medical Physics |
||
The 2011 Laughlin Symposium will focus on cutting-edge methods or devices designed to enhance, quantify, and/or monitor the safe use of radiation in diagnostic and therapeutic medicine, with emphasis on innovative science and engineering for patient safety. The timeliness of such work is evident in sensational coverage in the lay press reporting of accidents involving radiation in both the diagnostic and radiation therapy sectors. As a forum for high-quality, innovative scientific research, the symposium seeks abstract submissions on topics that go beyond basic quality control standards in radiation safety and identify new avenues for assuring patient safety. NOTE: Those wishing to be considered for the John S. Laughlin Science Council Research Symposium MUST submit abstracts to this category only. Proffered submission: Authors interested in being considered for the Symposium MUST:
Junior Investigator Competition participants who also wish to be considered for the Science Council Research Symposium should consider the following:
Young Investigator Competition participants:
All abstracts submitted to the John S. Laughlin Science Council Research Symposium that are NOT selected for the symposium, will be considered for oral or standard poster presentation.
|
||
| Computed Tomography | ||
| Cone-Beam CT | For abstracts with primary focus in guidance of radiation treatment delivery (e.g., localization in IGRT) or treatment planning. Topics related to cone-beam CT, including the use of flat-panel detectors for cone-beam CT. | |
| Fluoroscopy | ||
| For abstracts with primary focus in application to radiation treatment delivery (e.g., IGRT) or motion management application (e.g., therapy planning). Topics related to real-time fluoroscopy, including digital techniques, imaging system design and characterization, new methods of performance characterization, and clinical studies. | ||
| Image Registration, Fusion, Segmentation, and Visualization | ||
| Image Registration and Fusion | For abstracts with primary focus in treatment planning and guidance (e.g., IGRT). Techniques for registration and/or fusion of image data, including any combination of (2D, 3D, and/or 4D) imaging modalities, deformable modeling, and related topics. | |
| Image Segmentation | For abstracts with primary focus in treatment planning and guidance (e.g., IGRT). Topics in all areas of medical image processing including new methodologies, image or feature enhancement, tissue segmentation, and clinical evaluation, as applied to any medical imaging modality. | |
| Image Visualization | For abstracts with primary focus in treatment planning and guidance (e.g., IGRT). Techniques for rendering and display of medical image data. | |
| Magnetic Resonance Imaging | ||
| New Technology and Techniques (JOINT) | Abstracts related to the development or application of MRI, MRS, and/or MRA in radiation therapy planning and/or delivery. | |
| Outcome modeling | ||
| Imaging for Therapy Assessment (JOINT) | Abstracts related to image-based measurement or modeling of normal and tumor tissue response / treatment efficacy. | |
| Patient Simulation Imaging for Planning | ||
| Imaging for patient simulation: initial treatment planning, mid-treatment intervention and followup assessment of therapy. Includes all imaging modalities, CT, MR, PET, etc. | ||
| Non-moving Targets | Imaging for planning, non-4D imaging of "rigid" targets, e.g. brain, head/neck, spine, GU/GYN. | |
| Moving Targets | Imaging for planning, 4D imaging of "moving" targets, including 4D imaging for tumors in the thorax, liver, and other GI/abdominal sites. | |
| Cone-Beam CT | ||
| Others | ||
| Contouring and Organ Segmentation and Assessment of Margins | Definition of contours for tumors and normal tissues, including novel methods for segmentation using deformable image registration and other tools. Definition of margins for Planning. | |
| Anatomical Imaging | Use of imaging to define contours for tumors and normal tissues based on anatomical features, e.g. conventional CT and MR imaging. | |
| Functional and Molecular Imaging | Use of imaging to define contours for tumors and normal tissues based on functional and molecular features, e.g. dynamic contrast-enhanced MR or CT, functional PET. | |
| Image Segmentation | Use of imaging to define contours for tumors and normal tissues based on image segmentation. | |
| Image Registration/Fusion | (Image registration for intra- and inter-modality imaging, e.g. CT-to-CT, CT-to-CBCT, CT-to-MR, including rigid and deformable techniques. Not including novel methods for tumor and normal organ segmentation. | |
| Image Visualization | Image Visualization for planning. | |
| Treatment Delivery - Immobilization and Localization | ||
| Includes all aspects of immobilization and localization. | ||
| Patient Immobilization and Localization | Techniques for immobilizing and localizing the patient during treatment delivery. | |
| Immobilization Techniques | Specific devices/techniques used for patient immobilization. | |
| Tomographic Localization | Cone-beam CT (CBCT) artifact reduction, CBCT HU correction, limited projection techniques, MVCT, etc. | |
| Non-tomographic Localization | Management of patient motion during delivery using gating approaches and tumor tracking technologies. | |
| Motion Management (Adaptive Delivery) |
Management of patient motion during delivery using adaptive techniques, such as daily, on-line correction, on-line adaptive replanning and treatment. | |
| Motion Management (Gating/Tracking) | Management of patient motion during delivery using gating approaches and tumor tracking technologies. | |
| Targeted radionuclide therapies | ||
| Category | Description | |
|---|---|---|
| Patient Simulation Imaging for Planning (JOINT) | ||
| Imaging for patient simulation: initial treatment planning, mid-treatment intervention and followup assessment of therapy. Includes all imaging modalities, CT, MR, PET, etc. | ||
| Non-moving Targets | Imaging for planning, non-4D imaging of "rigid" targets, e.g. brain, head/neck, spine, GU/GYN. | |
| Cone-Beam CT | ||
| Others | ||
| Moving Targets | Imaging for planning, 4D imaging of "moving" targets, including 4D imaging for tumors in the thorax, liver, and other GI/abdominal sites. | |
| Contouring and Organ Segmentation and Assessment of Margins | Definition of contours for tumors and normal tissues, including novel methods for segmentation using deformable image registration and other tools. Definition of margins for Planning. | |
| Anatomical Imaging | Use of imaging to define contours for tumors and normal tissues based on anatomical features, e.g. conventional CT and MR imaging. | |
| Functional and Molecular Imaging | Use of imaging to define contours for tumors and normal tissues based on functional and molecular features, e.g. dynamic contrast-enhanced MR or CT, functional PET. | |
| Image Segmentation | Use of imaging to define contours for tumors and normal tissues based on image segmentation. | |
| Image Registration/Fusion | (Image registration for intra- and inter-modality imaging, e.g. CT-to-CT, CT-to-CBCT, CT-to-MR, including rigid and deformable techniques. Not including novel methods for tumor and normal organ segmentation. | |
| Image Visualization | Image Visualization for planning. | |
| Treatment Planning | ||
| Includes all aspects of treatment planning for external beam therapy (EBT), stereotactic radiosurgery and body radiotherapy (SRS and SBRT) and brachytherapy. | ||
| 3D Planning Including Adaptive Approaches | 3D-CRT planning for all sites including off-line adaptive planning and replanning. | |
| External Beam Therapy (EBT) | 3D-CRT planning for all sites including off-line adaptive planning and replanning for EBT. | |
| SRS/SBRT | 3D-CRT planning for all sites including off-line adaptive planning and replanning for SRS/SBRT. | |
| Brachytherapy | 3D-CRT planning for brachytherapy including LDR and HDR applications. | |
| Intensity Modulated Planning Including Adaptive Approaches | IMRT planning for all sites including off-line adaptive planning and reoptimization. | |
| External Beam Therapy (EBT) | IMRT planning for all sites including off-line adaptive planning and replanning for EBT. | |
| SRS/SBRT | IMRT planning for all sites including off-line adaptive planning and replanning for SRS/SBRT. | |
| Brachytherapy | IMRT planning for brachytherapy including LDR and HDR applications. | |
| Optimization Techniques | Optimization techniques and algorithms for treatment planning for EBT, SRS/SBRT and Brachytherapy. | |
| Biological Modeling | Use of biological models and dose indices in treatment planning, e.g. EUD. | |
| Dose Calculations - All Modalities | Dose calculation algorithms for treatment planning, all sites. | |
| Monte Carlo Techniques | Monte Carlo-based dose calculation for linac simulation and treatment planning. | |
| Non-Monte Carlo Techniques | Other, non-Monte Carlo-based dose calculation algorithms for linac simulation and treatment planning for EBT and brachytherapy. | |
| Treatment Delivery and Verification | ||
| Includes all aspects of treatment delivery and related verification. | ||
| Patient Immobilization and Localization (JOINT) | Techniques for immobilizing and localizing the patient during treatment delivery. | |
| Immobilization Techniques | Specific devices/techniques used for patient immobilization. | |
| Tomographic Localization | Cone-beam CT (CBCT) artifact reduction, CBCT HU correction, limited projection techniques, MVCT, etc. | |
| Non-tomographic Localization | Management of patient motion during delivery using gating approaches and tumor tracking technologies. | |
| Motion Management (Adaptive Delivery) |
Management of patient motion during delivery using adaptive techniques, such as daily, on-line correction, on-line adaptive replanning and treatment. | |
| Motion Management (Gating/Tracking) | Management of patient motion during delivery using gating approaches and tumor tracking technologies. | |
| 3D Delivery Techniques | 3D-CRT delivery techniques and related verification measurements. | |
| Photons | 3D-CRT delivery techniques and related verification methods for photons. | |
| Electrons | 3D-CRT delivery techniques and related verification methods for electrons. | |
| Protons | 3D-CRT delivery techniques and related verification methods for protons. | |
| Other Charged Particles | 3D-CRT delivery techniques and related verification methods for other charged particles, e.g. Carbon ions. | |
| Intensity Modulated Delivery Techniques | IMRT delivery techniques and related verification measurements. | |
| Photons | IMRT delivery techniques and related verification methods for photons. | |
| Electrons | IMRT delivery techniques and related verification methods for electrons. | |
| Protons | IMRT delivery techniques and related verification methods for protons. | |
| Brachytherapy | Includes all aspects of brachytherapy delivery and verification. | |
| HDR Techniques | Includes all aspects of brachytherapy delivery and verification for HDR applications. | |
| LDR Techniques | Includes all aspects of brachytherapy delivery and verification for LDR applications. | |
| SRS and SBRT | Includes all aspects of SRS/SBRT delivery and verification. | |
| Patient Safety and Quality Assurance (QA) Procedures | ||
| Includes all aspects of patient safety and quality assurance. | ||
| Devices and Detectors for Measurements | Devices and detectors used for QA measurements. | |
| Off-line | Devices and detectors used for off-line measurements, e.g. daily and monthly QA tools, and patient-specific QA devices. | |
| On-line | Devices and detectors used for on-line measurements. | |
| Calculation Tools | Calculation tools for QA. | |
| Safety Procedures | Safety procedures for patient safety and QA. | |
| Outcome Modeling | ||
| Includes all aspects of patient outcomes modeling. | ||
| Imaging for Therapy Assessment (JOINT) | Abstracts related to image-based measurement or modeling of normal and tumor tissue response / treatment efficacy. | |
| Late Effects | Outcomes modeling based on late effects. | |
| Early Effects | Outcomes modeling based on early effects. | |
| Basic Radiobiology | ||
| Includes all aspects of basic radiobiological experiments and modeling. | ||
| Informatics | ||
| Includes all aspects of informatics in imaging and radiotherapy. | ||
| Small Animal Studies | ||
| Radiation Protection and Shielding | ||
| Includes all aspects of radiation protection and shielding. | ||
| targeted radionuclide therapies | ||
| Category | Description | |
|---|---|---|
| Ultrasound Imaging | ||
| Guidance of Interventions | Image guided surgery, noninvasive ablation, radiation therapy, biopsy, homeostasis, vascular and joint puncture, neural stimulation, sterilization, use of image registration for guidance. | |
| Advanced Systems and Components, Evaluations and Safety | Systems, transducers and scan heads, beamforming, advances in 3D imaging and display, portable systems and world health. System performance evaluation, image quality, quality control, modeling effect on clinical studies, health impact, cost of not performing study/developing systems, bioeffects. | |
| Signal/Image Processing & Display Algorithms | Speckle reduction, aberration correction, wave migration, super resolution, CAD, image registration, modeling | |
| Quantitative Imaging & Measures | Scatterer size and density, speed of sound, attenuation and nonlinearity parameters, impedance, temperature, permeability, bone stability, tissue/lesion volumes, fundamental physics and chemistry, and tissue/fluid motion--Doppler, speckle tracking, small vessel and volume flow, strain & elastic modulus, shear wave velocity, pulsatility, current source, slip boundaries | |
| Contrast and Interventional Agents (US & Photoacoustic) | Targeted or acoustically selected agents, their medical and research uses and trials thereof | |
| Small Animal Imaging | Systems and applications | |
| Breast and Other Applications | Breast Imaging, other applications, 20 MHz to terahertz imaging, other US/multimodality imaging, research support. | |
| Photoacoustic Imaging | Technology, Algorithms, Applications, Sources – lasers, diodes. Sensors – optical, piezoelectric. Systems – imaging and measurement. Safety; Advanced image reconstruction, limited apertures, spectral analysis, signal processing, quantification; Small animal, microscopy, functional and molecular imaging. Medical - intravascular, breast, skin, endoscopic, arthritis, ophthalmic, prostate, treatment monitoring & assessment | |
| Therapeutic Ultrasound advances | ||
| High-Intenstiy Therapetic Ultrasound Devices | ||
| HIFU | Topics related to High Intensity Focused Ultrasound (HIFU) technology, including MR and ultrasound guided systems. | |
| Other HITU Devices | Topics related to other High-Intensity Therapeutic Ultrasound (HITU) devices and technology: includes percutaneous, intraluminal,catheter-based, endocavity, laparoscopic, endoscopic, and external devices; treatment of cancer and non-oncological tissues; standalone or combined with image guidance. | |
| Advanced and Novel Delivery Systems | ||
| Thermal Therapy | Therapeutic ultrasound applied to hyperthermia and thermal ablation for cancer therapy; non-oncological interventions including treatment of benign disease, hemostasis, nerve block, tissue remodeling & cosmesis. | |
| Non-thermal Therapies | Topics for generating non-thermal mediated treatments with ultrasound, such as using acoustic processes such as cavitation, radiation force, and acoustic drug activation and delivery; includes wound/bone healing, control of tissue development, tissue regeneration, blood/brain barrier disruption, & drug delivery | |
| Small Animal Systems | Topics related to small animal systems and tumor models for investigating therapetic ultrasound | |
| Novel Approaches | Novel approaches for delivery or control of treatment; new treatment strategies | |
| Commercial Systems | Description of system performance and capabilities of commercial systems from a scientific perspective. | |
| Image Guidance and Assessment of Ultrasound Therapies | ||
| MR Techniques | Topics related to MRI for treatment guidance, monitoring, and assessment of treatment delivery including: MR techniques for temperature monitoring, treatment verification or setup using MR ARFI, diffusion wieghted techniques, & contrast enhanced imaging, novel pulse sequences, and motion compensation. | |
| US Techniques | Topics related to ultrasound imaging for treatment guidance, monitoring, and assessment of treatment delivery including: temperature monitoring,change in backscatter, tissue changes during therapy, CEUS, Doppler, elastography and shear wave imaging for thermal lesion assessment or evidence of tissue modification. | |
| CT, X-ray, Fluoroscopy and Other Techniques | Topics related to guiding and monitoring ultrasound therapies using CT ,fluoroscopy techniques, IR and optical approaches. | |
| Treatment Planning & Modeling for Ultrasound Therapeutic Intervention | ||
| Treatment Planning & Optimization | Topics related to acoustic and biothermal treatment planning, optimization based approaches applied to minimizing treatment duration, improving localization, thermal dose, drug delivery, and ultrasound therapy delivery | |
| Modeling and Control | Topics related to biothermal and acoustic model development, pharmacokinetic drug delivery models, thermal dose and thermal damage models. Methods of temperature and other types of feedback control of treatment. | |
| Bubble Based Ultrasound Therapies | ||
| Bubbles for Therapy | Topics and techniques related to bubbles for therapy enhancement and localization, including mechanisms and ability to control, possible tissue protection or preferential targeting, non-thermal therapies. | |
| Drug Delivery, Activation and Enhancement | ||
| Nanoparticles & Therapeutic Agents | Topics related to nanoparticles, nanoemulsions, and biological agents that are activated or localized/released by therapeutic ultrasound; acoustic or thermal mediated processes; chemotherapy, gene therapy, and immunotherapy | |
| Ultrasound Therapy Program and HIFU/HITU Standards | ||
| Quality Assurance & Standards | Topics and techniques for the development of quality assurance measurement tools and procedures for safety and quality control of ultrasound therapetic devices. | |
| Ultrasound Therapy Program | Topics and elements in support of development of an ultrasound therapy program, including FDA regulations, NIH support, and reimbursement issues. | |
| Biological & Physiological Effects of Therapeutic Ultrasound | ||
| Bioeffects | All aspects of biological and physiological effects of therapetic ultrasound as modified or altered in various treatment regimens as a means to improve therapy delivery; includes radiosensitization and chemosensitization, permeability and extravasation, opening of the blood brain barrier, immune response, increase oxygenation, temporary nerve block, and tissue remodeling. | |
| Clinical Ultrasound Therapy | ||
| Clinical Implementation and Studies | All aspects of clinical implementation of Pilot and Phase I/II/III studies of ultrasound therapy systems. Examples include treatment of uterine fibroids, palliative treatment for bone tumors, localized prostate cancer, renal tumors, esophageal cancer, brain neoplasms; includes initial feasibility and performance data; alone or combined with radiation and/or chemotherapy. | |
| Category | Description | |
|---|---|---|
| Other Physics and Biomedical Engineering | ||
| Abstracts pertaining to topics of medical physics or biomedical engineering not covered in the Imaging, Therapy, Joint, and Ultrasound tracks detailed above. Examples include development or clinical application of new biophysics research, prosthetic devices, tissue engineering techniques, general medical devices, surgical robotics, etc. | ||





